- Radical S-Adenosyl-l-Methionine Enzyme PylB: A C-Centered Radical to Convert l-Lysine into (3R)-3-Methyl-d-Ornithine. Soualmia F, Cherrier MV, Chauviré T, Mauger M, Tatham P, Guillot A, Guinchard X, Martin L, Amara P, Mouesca JM, Daghmoum M, Benjdia A, Gambarelli S, Berteau O, Nicolet Y. J Am Chem Soc. 2024 . doi: 10.1021/jacs.3c03747.
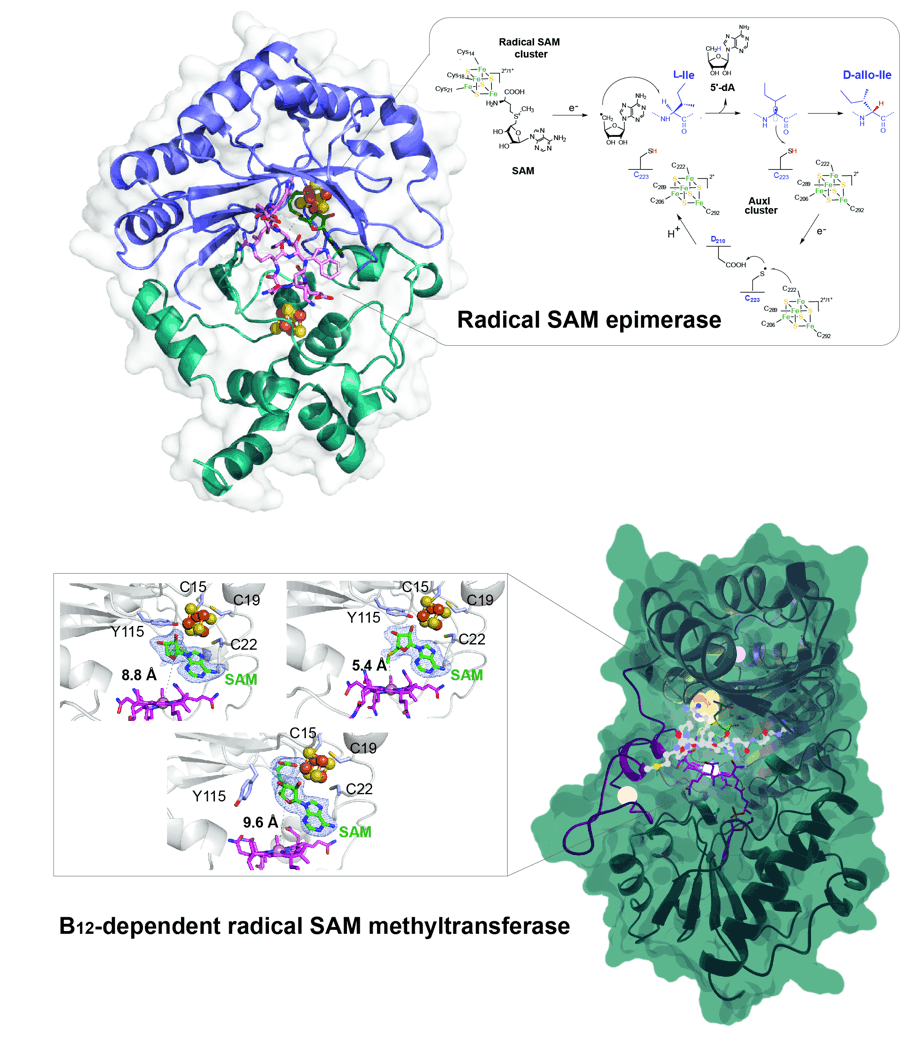
- Structural and mechanistic basis for RiPP epimerization by a radical SAM enzyme. Kubiak X, Polsinelli I, Chavas LMG, Fyfe CD, Guillot A, Fradale L, Brewee C, Grimaldi S, Gerbaud G, Thureau A, Legrand P, Berteau O, Benjdia A. Nat Chem Biol. 2024 Mar;20(3):382-391. doi: 10.1038/s41589-023-01493-1.
- B12-dependent radical SAM enzymes: Ever expanding structural and mechanistic diversity. Benjdia A, Berteau O. Curr Opin Struct Biol. 2023 Dec;83:102725. doi: 10.1016/j.sbi.2023.102725.
- Crystallographic snapshots of a B12-dependent radical SAM methyltransferase. Fyfe CD, Bernardo-García N, Fradale L, Grimaldi S, Guillot A, Brewee C, Chavas LMG, Legrand P, Benjdia A, Berteau O. Nature. 2022 Feb;602(7896):336-342. doi: 10.1038/s41586-021-04355-9.
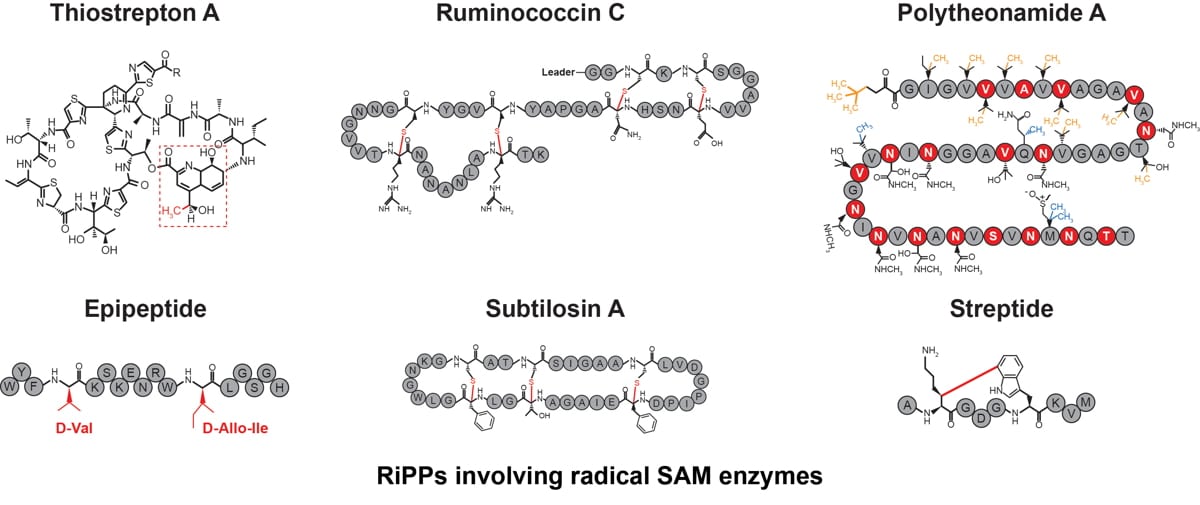
- Biosynthesis of the sactipeptide Ruminococcin C by the human microbiome: Mechanistic insights into thioether bond formation by radical SAM enzymes. Balty C, Guillot A, Fradale L, Brewee C, Lefranc B, Herrero C, Sandström C, Leprince J, Berteau O, Benjdia A. J Biol Chem. 2020 Dec 4;295(49):16665-16677. doi: 10.1074/jbc.RA120.015371.
- Mechanistic Investigations of PoyD, a Radical S-Adenosyl-l-methionine Enzyme Catalyzing Iterative and Directional Epimerizations in Polytheonamide A Biosynthesis. Parent A, Benjdia A, Guillot A, Kubiak X, Balty C, Lefranc B, Leprince J, Berteau O. J Am Chem Soc. 2018 Feb 21;140(7):2469-2477. doi: 10.1021/jacs.7b08402.
- Post-translational modification of ribosomally synthesized peptides by a radical SAM epimerase in Bacillus subtilis. Benjdia A, Guillot A, Ruffié P, Leprince J, Berteau O. Nat Chem. 2017 Jul;9(7):698-707. doi: 10.1038/nchem.
- Carbon-sulfur bond-forming reaction catalysed by the radical SAM enzyme HydE. Rohac R, Amara P, Benjdia A, Martin L, Ruffié P, Favier A, Berteau O, Mouesca JM, Fontecilla-Camps JC, Nicolet Y. Nat Chem. 2016 May;8(5):491-500. doi: 10.1038/nchem.2490.
- The thiostrepton A tryptophan methyltransferase TsrM catalyses a cob(II)alamin-dependent methyl transfer reaction. Benjdia A, Pierre S, Gherasim C, Guillot A, Carmona M, Amara P, Banerjee R, Berteau O. Nat Commun. 2015 Oct 12;6:8377. doi: 10.1038/ncomms9377.
- Thiostrepton tryptophan methyltransferase expands the chemistry of radical SAM enzymes. Pierre S, Guillot A, Benjdia A, Sandström C, Langella P, Berteau O. Nat Chem Biol. 2012 Dec;8(12):957-9. doi: 10.1038/nchembio.1091.
All the team publications are available in the CHEMSYBIO-MICALIS HAL collection.