BaPS
Pathogenic Bacteria and Health
Our team is working on the pathophysiology of Clostridioides difficile infections, an anaerobic, spore-forming Gram-positive bacterium that is a major enteropathogen in human and animal health. C. difficile has been classified as an urgent threat in terms of antibiotic resistance by the CDC in 2019 (Centers for Disease Control and Prevention (U.S.), 2019). In this context, our team’s objectives are to gain a better understanding of the pathophysiology of C. difficile infections in order to identify ways of combating this intestinal pathogen. Four lines of research are currently being developed to study this bacterium:
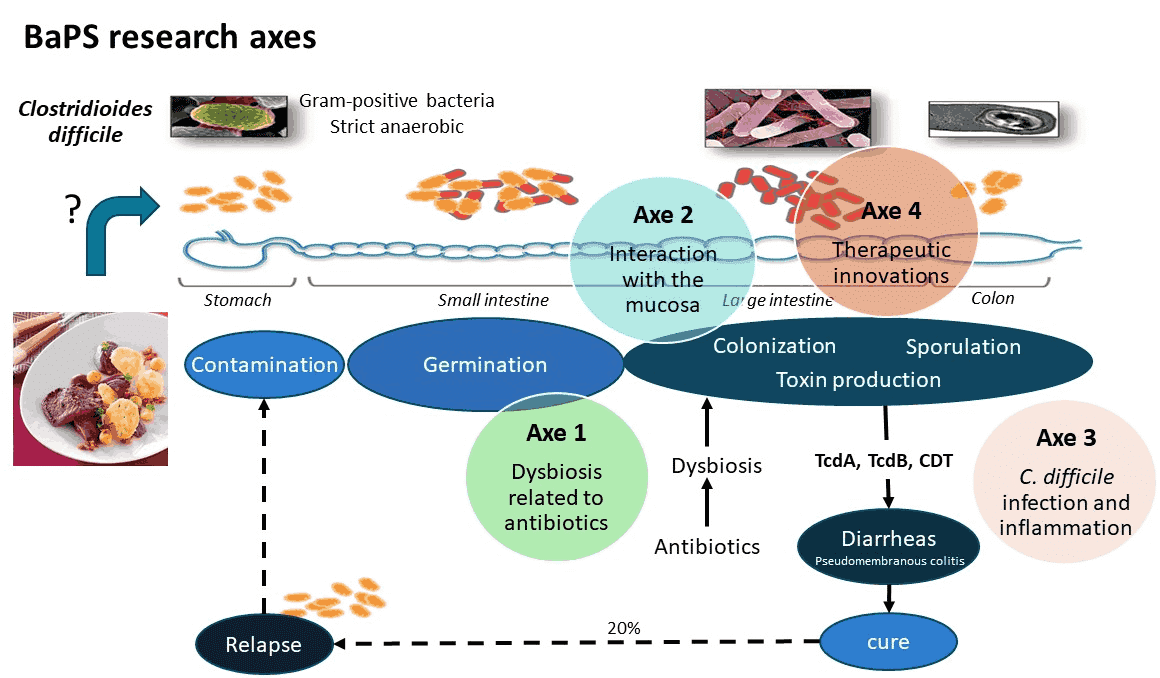
Clostridioides difficile is responsible for a large proportion of post-antibiotic diarrhoea and most pseudomembranous colitis in humans and of many intestinal infections in livestock. The first step of C. difficile infection (CDI) corresponds to the colonization of the host. This step is made possible by a dysbiosis of the microbiota, following most of the time antibiotic exposure. The resulting disruption of the barrier effect favors germination of the infectious spores and implantation of C. difficile in its intestinal niche. Clinical signs arise from the effect of the toxins (main virulence factors) on intestinal cells and tissues. Infection outcome is known to be related to the strain virulence but also to the intensity of the host response, both innate and adaptive. Indeed, inflammatory response, essential for the pathogen clearance, may also exert deleterious effect for the host if uncontrolled. CDI is treated by antibiotics but recurrent infections occur frequently, leading in some patients to multiple recurrences experiences, that are relapses for most of the time. The persistence of the initial strain in the patient, in an asymptomatic colonization status, has been long time related to spore production, but recently the hypothesis of persistence of C. difficile within a biofilm was also proposed.
With more than 20 years of research into C. difficile, our team is recognized to have a great expertise on C. difficile pathophysiology and CDI. Our objectives are both to go on increasing basic knowledge on the interactions between C. difficile and the host and to identify new strategies to fight against CDI. Several projects have been developed to address these questions: the mechanisms underlying the increase of infectious risk following dysbiosis of the intestinal microbiota; the study of C. difficile biofilm formation and architecture to understand interactions with the digestive mucosa; the characterization of several important polymeric components of the bacterial surface (e.g. flagella, polysaccharides, peptidoglycan) as new targets for C. difficile inhibition; the study of the innate (and its regulation) and adaptive host response to virulence factors; development of therapeutic innovations such as mucosal vaccines or probiotics. These projects are grouped into 4 thematic axes (see below).
To carry out these projects, the unit currently comprises, as permanent staff, 12 teachers-researchers, 2 technicians (research and teaching) and a secretary. A third technician (research and teaching) and one laboratory aid are present as fixed-term paid contract funded by the pharmaceutical Faculty. We are currently supervising 3 PhD, 3 M2 students, 3 research engineers and one assistant engineer under own resources (external contracts). We have a variety of tools and expertises, in anaerobic bacteria, molecular bacteriology and genetic manipulation of Clostridia, cellular microbiology, medical microbiology, and rodent CDI models.
Research axis
Antibiotics, dysbiosis and emergence of infectious risk (ATB-DRI)
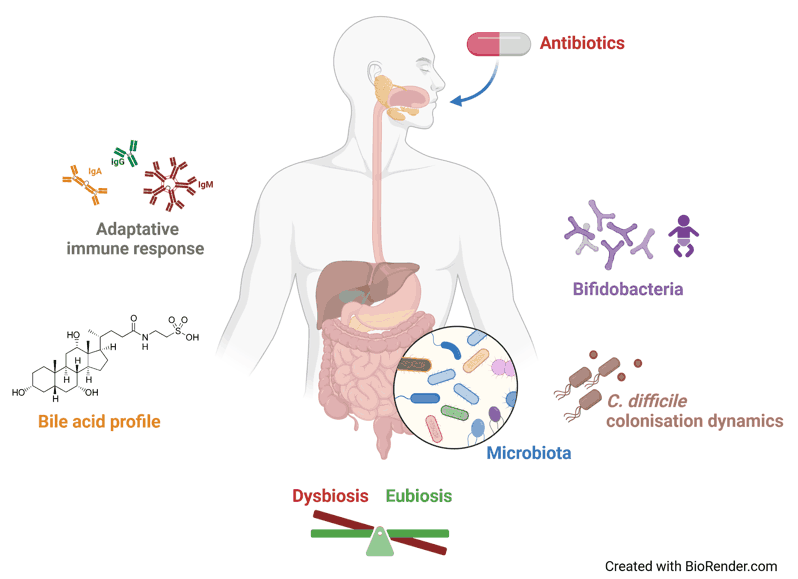
The ATB-DRI group investigates the impact of antibiotics, especially the gut microbiota dysbiosis, on the emergence of infections, in particular C. difficile infections (CDI).
We study factors influencing gut colonization process, clearance, or persistence of C. difficile, and how C. difficile interacts with different digestive microenvironments and the host. We use experimental model of CDI in mice and ex vivo devices, simulating the conditions of the digestive tract, to study microbial signatures, bile acid metabolism, and mucosal immune response. Our project also integrates a translational approach in humans, with clinically documented collections from cohorts of adult and infant, to develop targeted therapeutics and prevention strategies against CDI (especially involving bifidobacteria species).
Interaction of C. difficile with the mucosa
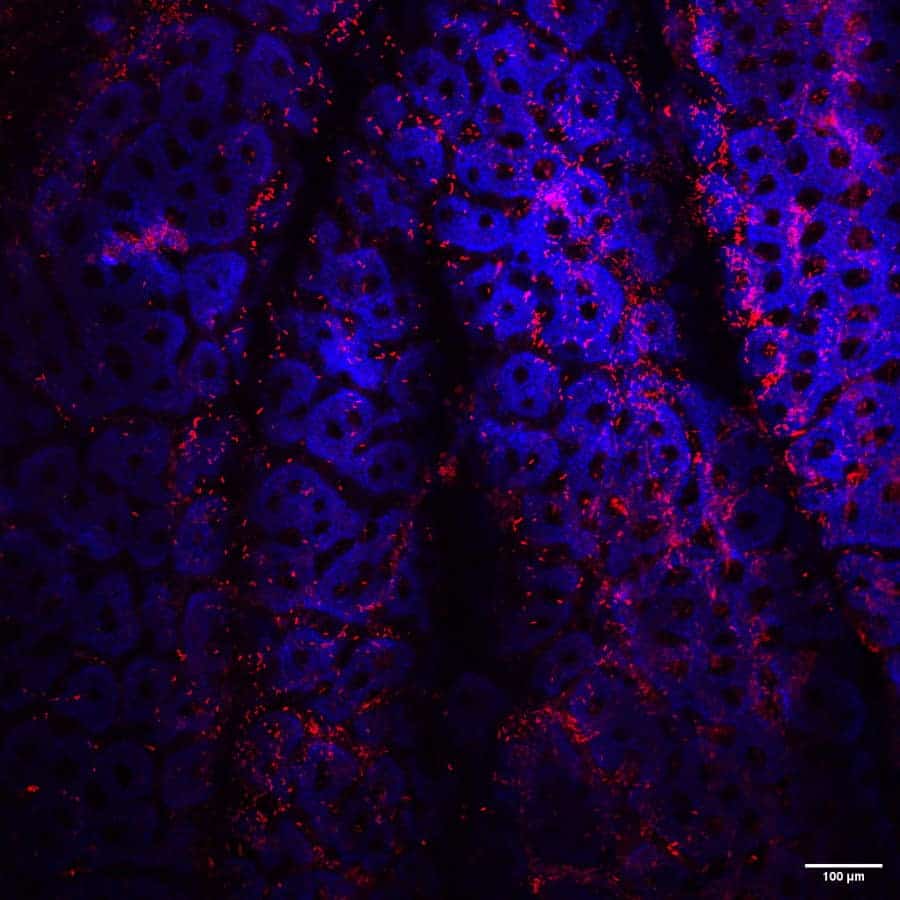
This axis currently focusses on biofilms formed by C. difficile, both in vitro to understand biofilm production and main characteristics, and in vivo, to explore the putative role of C. difficile biofilms in the occurrence of relapses. In vitro, we are particularly working on the role of extracellular DNA in the biofilm formation and properties of C. difficile and on the identification of factors able to trigger the production of biofilm (notably the sub-inhibitory concentrations of β-lactam antibiotics). In vivo, we are interested in the identification of markers of biofilm formation. We also work on mixed-biofilm formation between CD and specific commensal bacteria in gnotobiotic mouse model.
C. difficile infection and inflammation
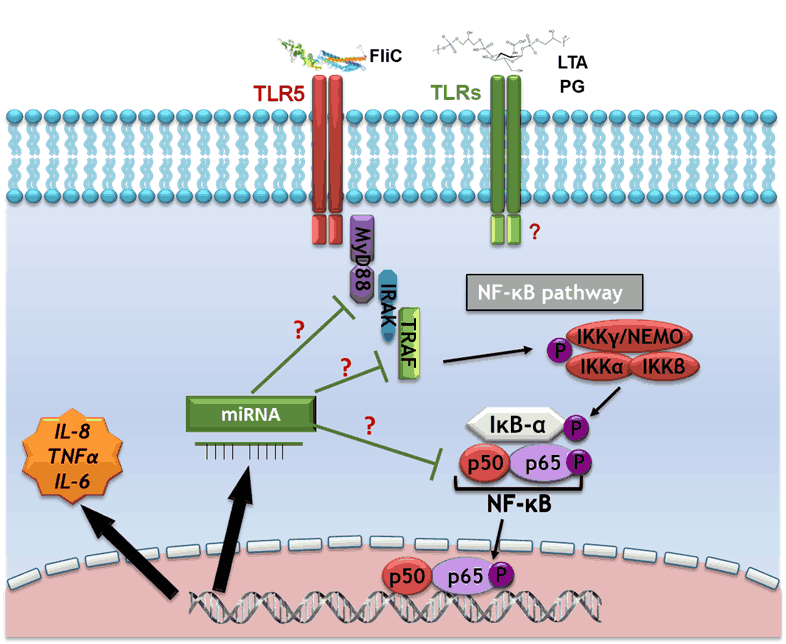
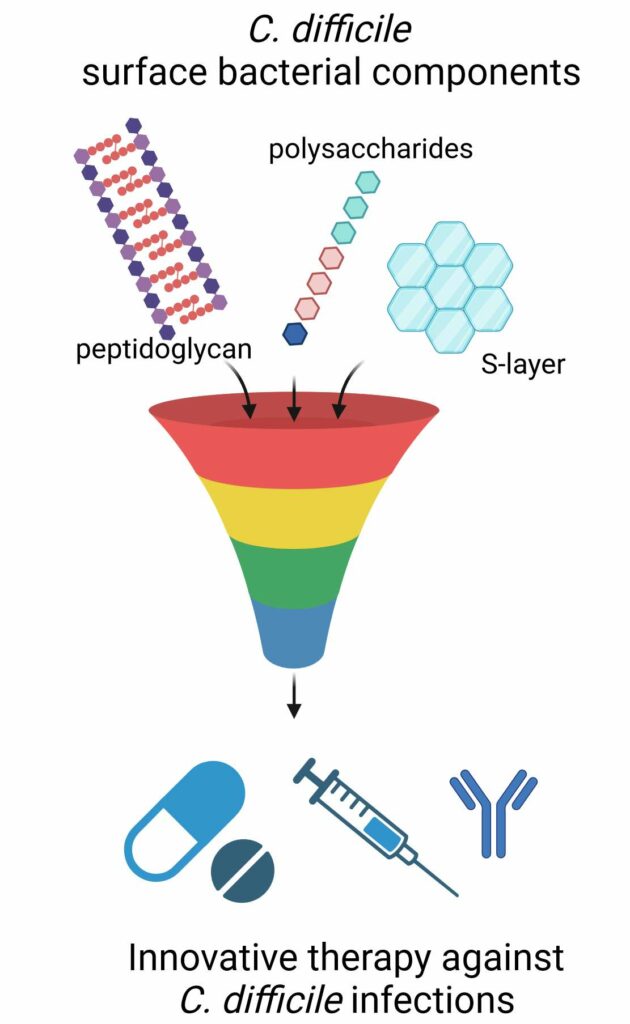
We focus our research on C. difficile flagella and other surface components of the bacterium, which can be potentially involved in the development of an inflammatory host response and could constitute new therapeutic targets for C. difficile infections. We demonstrated that TLR5-flagellin interaction elicits signaling pathways involving MAPKs and NF-kB, which are responsible for the secretion of pro-inflammatory cytokines. The lipoteichoic acids, and the peptidoglycan of C. difficile could also be involved in the pro-inflammatory response of the host. The effectors of the signaling pathways involved in host response against these compounds and the potential pro- or anti-inflammatory regulatory role of miRNAs are currently under investigation.
Therapeutic innovations
Team members
Alumni
- Liste à puce
- Liste à puce
- Liste à puce
- Liste à puce
- Liste à puce
- Liste à puce
- Liste à puce
Key points
- Microbiol Spectr 2023 Feb 23;11(2):e0422722. Villemagne et al. Polysaccharide II surface anchoring, the Achilles’ heel of Clostridioides difficile
- Antibiotics 2022 May 5;11(5):624. Doan et al. Impact of Subinhibitory Concentrations of Metronidazole on Morphology, Motility, Biofilm Formation and Colonization of Clostridioides difficile.
- Hosp Infect 2022 Nov:129:65-74. Le Monnier et al. One-day prevalence of asymptomatic carriage of toxigenic and non-toxigenic Clostridioides difficile in 10 French hospitals.
- Front Microbiol 2017 Front Microbiol Oct 25:8:2086. Soavelomandroso et al. Biofilm Structures in a Mono-Associated Mouse Model of Clostridium difficile Infection.
- Sci Rep 7(1): 3256 Batah et al. Clostridium difficile flagella induce a pro-inflammatory response in intestinal epithelium of mice in cooperation with toxins.
- J Hosp Infect 2022 Nov:129:65-74. Le Monnier et al. One-day prevalence of asymptomatic carriage of toxigenic and non-toxigenic Clostridioides difficile in 10 French hospitals.
- Doan et al. 2022 May 5;11(5):624. Impact of Subinhibitory Concentrations of Metronidazole on Morphology, Motility, Biofilm Formation and Colonization of Clostridioides difficile.
All the team publications are available in the BAPS-MICALIS HAL collection
- International patent PCT/EP2015/05725 (in collaboration)
- International Patent PCT/EP2019/080748
- Maturation project SATT Mir, MicroRNA for the treatment of intestinal inflammation, (2021-2024), Coordinator
- POC-in-Lab MIRCa (2023-203), funded by GS HeaDS (IDEX)
- ANR DifKin (ANR-17-CE15-0018): A Serine/threonine kinase promotes resistance to antimicrobial coumpounds crucial during colonization in Clostridium difficile (2018-2021), Partner
- ANR Dalatar (ANR-19-CE18-0008): Targeting D-alanylation of teichoic acids to fight clinically-problematic Gram-positive pathogens (2020-2024), Partner
- ANR DifBioRel (ANR-20-CE15-0022): Exploring biology of biofilm formation by Clostridium difficile and its role in gut persistence and relapse of infection (2021-2025), Partner
- ANR CdiffRib (ANR-22-CE15-0020): Role of RNA-dependent control in the pathophysiology of Clostridioides difficile and its interaction with the host (2023-2026), Partner,
- ANR DifOx (ANR-22-CE15-0026): Effect of exposure to oxygen and oxidative stress on Clostridioides difficile gut colonization and virulence (2023-2026), Partner,
- ANR TympaBiOM (ANR-22-CE52-0006) : Biomatériaux actifs contenant des probiotiques pour la régénération de la membrane tympanique dans l’otite moyenne chronique suppurée, (2023-2026), Partner,
- NR PSDIFF (ANR-23-CE18-0037): Clostridioides difficile polysaccharides as therapeutic targets, (2024-2027), Coordinator,
- Industrial contract with BIOCODEX, Study of the impact of Saccharomyces boulardii CNCM I-745 on the biofilm of Clostridium difficile (2018-2022)
- Industrial contract PREVADIF with MSD, Seroprevalence of antibodies raised against surface antigens and toxins of Clostridioides difficile, (2021-2023)
- Industrial contract with EXELIOM, Preventive effect of Faecalibacterium prausnitzii in Clostrioides difficile infection (2023-2024)
- Industrial contract FIVADIF with TILLOTS PHARMA, Differential impact of Fidaxomycin and Vancomycin on early and late digestive clearance of Clostridioides difficile in infected patients (2024-2025)