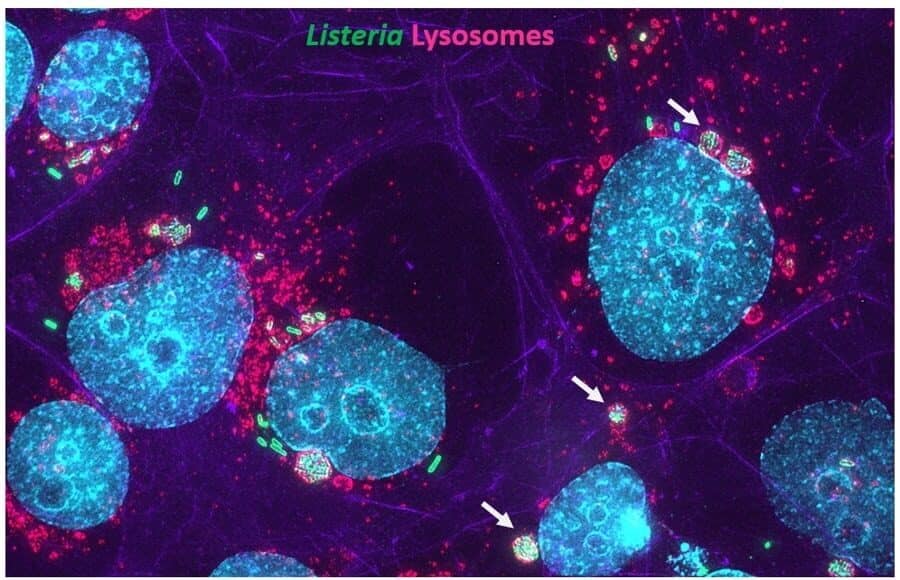
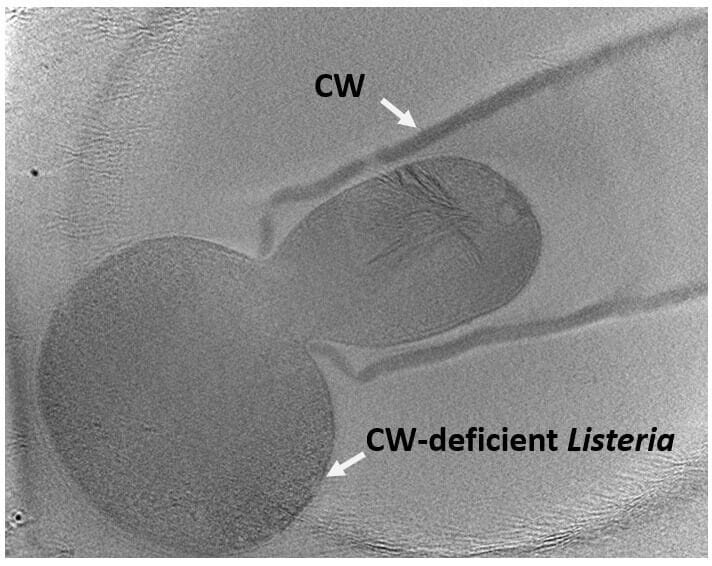
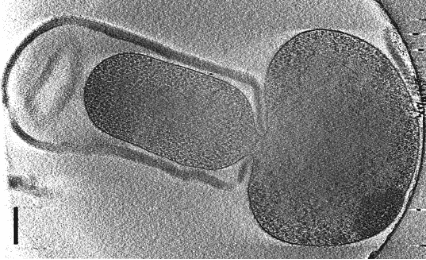
-
- B3D • Biofilms and Spatially Organized Communities
- BaPS • Pathogenic Bacteria and Health
- ComBac • Bacterial Communication
- CPE • Commensalism and Pathogenesis of Enterococci
- DynPhages • Bacteriophage Genome Dynamics
- EpiMiC • Epigenetics and Cellular Microbiology
- GME • Microbial Genetics and Environment
- MicrobAdapt • Determinants of Microbial Adaptation
- Paroi • Bacterial cell wall dynamics
- PIMs • Pathogens, Immunity and Microbiota
- B3D • Biofilms and Spatially Organized Communities
- BaPS • Pathogenic Bacteria and Health
- ComBac • Bacterial Communication
- CPE • Commensalism and Pathogenesis of Enterococci
- DynPhages • Bacteriophage Genome Dynamics
- EpiMiC • Epigenetics and Cellular Microbiology
- GME • Microbial Genetics and Environment
- MicrobAdapt • Determinants of Microbial Adaptation
- Paroi • Bacterial cell wall dynamics
- PIMs • Pathogens, Immunity and Microbiota
- AMIPEM • Food, Gut microbiota, Brain and Metabolic Diseases
- ChemSyBio • Biochemistry and Synthetic Biology
- FInE • Functionality of the Intestinal Ecosystem
- FME • Food Microbial Ecology
- MIHA • Microbiota Interactions with Human and Animal
- NutriPhage • Diet-driven dynamics of the gut virome
- PhylHom • Phylogeny and Physiology of the Human Microbiome
- ProbiHôte • Commensal and Probiotic Microorganisms-Host Interactions
- AMIPEM • Food, Gut microbiota, Brain and Metabolic Diseases
- ChemSyBio • Biochemistry and Synthetic Biology
- FInE • Functionality of the Intestinal Ecosystem
- FME • Food Microbial Ecology
- MIHA • Microbiota Interactions with Human and Animal
- NutriPhage • Diet-driven dynamics of the gut virome
- PhylHom • Phylogeny and Physiology of the Human Microbiome
- ProbiHôte • Commensal and Probiotic Microorganisms-Host Interactions
-