The pathogen Streptococcus pyogenes (Group A Streptococcus; GAS) causes severe infections with long-term sequellae. GAS infections account for 517,000 deaths annually worldwide. The research teams at the Cochin Institute (Bactéries et périnatalité; INSERM and CNRS) and at Micalis Institute (MicrobAdapt, INRAE) were intrigued by the presence of variants that emerged during experimental infections in primates, with significantly reduced virulence. How would this bacterium benefit from reduced virulence during infection? In a recent study published in Nature Communications, these teams combined their complementary expertise in medical microbiology (Cochin) and membrane lipid biology (Micalis). Indeed, the explanation for GAS variant emergence was found in its membrane!
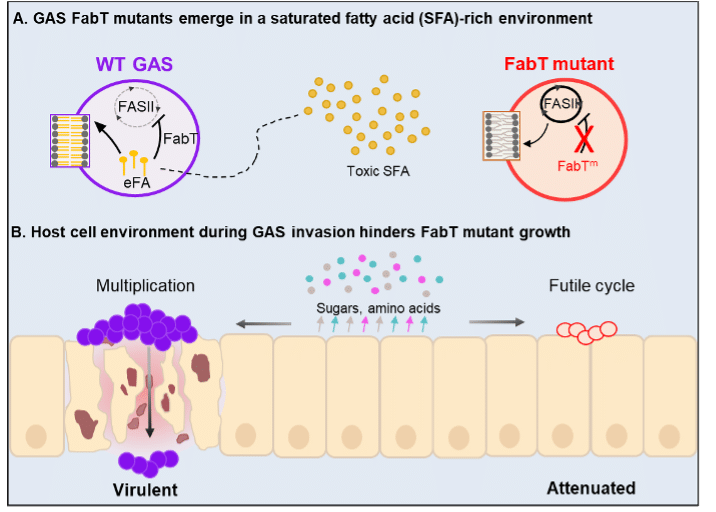
The FabT regulator controls synthesis of GAS fatty acids (FA), the essential building blocks of membrane lipids. FA are synthesized via the FASII pathway, or can be hijacked from host lipids. FabT regulates this ‘choice’. Typically, FabT blocks the FASII pathway to favor the use of host-derived FAs. In fabT mutants, however, the FASII pathway remains active, causing the bacterium to continuously synthesize FA. Notably, constant FA synthesis prevents uptake of potentially toxic host-derived FA, so the mutant survives better than the parent. But once infection is established, the mutants lose competitiveness, as the overactive FASII pathway leads to alteration of membrane composition, energy dissipation, halts bacterial growth, and ultimately leads to death of these GAS mutants.
This study elucidates the key role played by GAS lipid membranes in balancing survival and infection according to the infection site. It reveals how a deadly pathogen can lose its virulence through naturally-occurring point mutations in fabT during specific infection stages. It elucidates the underlying mechanism and the “double-edged” role of FabT depending on the host environment and lipid content. Moreover, this work opens new avenues for therapeutic targeting of FabT, offering potential for developing inhibitors and treatments against infections caused by GAS or other FabT-harboring pathogens such as Streptococcus agalactiae or Enterococcus faecalis.
Lambert, C., Gaillard, M., Wongdontree, P. et al. The double-edged role of FASII regulator FabT in Streptococcus pyogenes infection. Nat Commun 15, 8593 (2024).
Published October 4, 2024. The first author was awarded by the French Society for Biochemistry and Molecular Biology (Société Française de Biochimie et Biologie Moléculaire; SFBBM) for this publication.
Contact: Alexandra Gruss & Agnès Fouet
link: https://doi.org/10.1038/s41467-024-52637-3
Figure legend: Model for emergence of fabT mutants attenuated for virulence. A Saturated fatty acids (SFA) within a lipid environment favor FabT mutant emergence (FabTm). Toxic SFA may be present in initial GAS contacts with the host, and could select for emergence of FA-insensitive fabT mutants encoding FabTm, conferring a growth advantage. In a proof of concept, fabT mutants were selected in an SFA environment. B The nutrient-poor environment of the host cell during invasion hinders the growth of FabTm. Long-chain fatty acids, continuously produced by these mutants, destabilize GAS membranes and lead to futile metabolism, preventing bacterial growth. Thus, fabT mutants within GAS populations may confer a survival advantage at the inoculation site (rich in fatty acids) but do not withstand the nutrient-poor conditions of host infection. Mauve circles, WT; red circles, FabTm GAS; zoom is on phospholipids. Small yellow circles and lines, lipids and exogenous FA (eFA) products respectively. Small red, blue, pink circles, sugars and amino acids secreted by host cells (indicated by arrows).