First observation by electron microscopy of pairs of organised protofilaments and first three-dimensional structure of the bacterial homologue of MreB actin from a Gram-positive bacterium
Research into eukaryotic actin has historically focused on elucidating structure-function relationships from in vitro studies. However, purification of the prokaryotic actin MreB has proved particularly difficult, thwarting efforts to carry out in vitro studies. Instead, for twenty years research on MreB focused mainly on cell biology studies, stimulated by advances in fluorescence microscopy. Knowledge of the biochemical properties of MreB therefore remained very limited and based on MreBs from Gram-negative species.
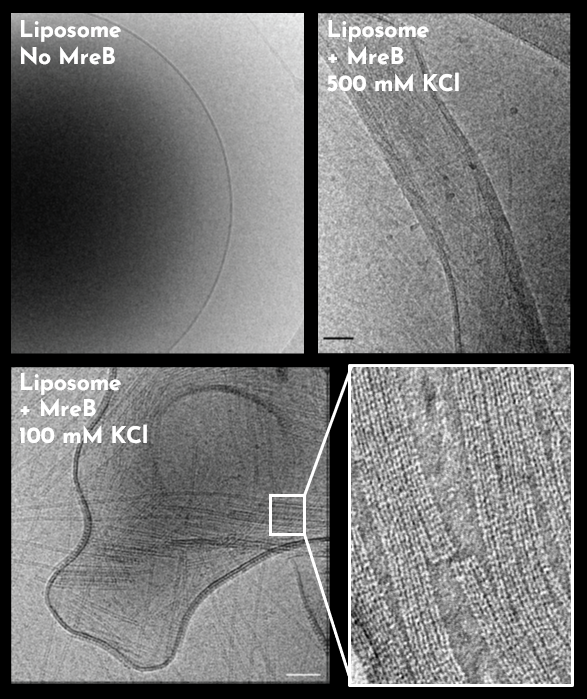
In a study published in eLife, scientists from the ProCeD team have crystallised and characterised the role of nucleotides and lipids in the polymerisation of MreB from the Gram-positive bacterium Geobacillus stearothermophilus. The authors showed that Geobacillus MreB forms pairs of straight protofilaments on the lipid surface in the presence of ATP/GTP, but not in the presence of ADP, GDP or non-hydrolysable ATP analogues. Membrane anchoring is mediated by two short hydrophobic sequences, while electrostatic interactions also contribute to lipid binding. The authors also showed that the population of membrane-bound protofilament doublets is in a stable dynamic state.
This study suggests that the assembly of Geobacillus MreB differs considerably from that of eukaryotic actin and that of MreB from Gram-negative bacteria. MreB assembly dynamics may therefore vary significantly between organisms.
Contact: Rut Carballido-López & Arnaud Chastanet