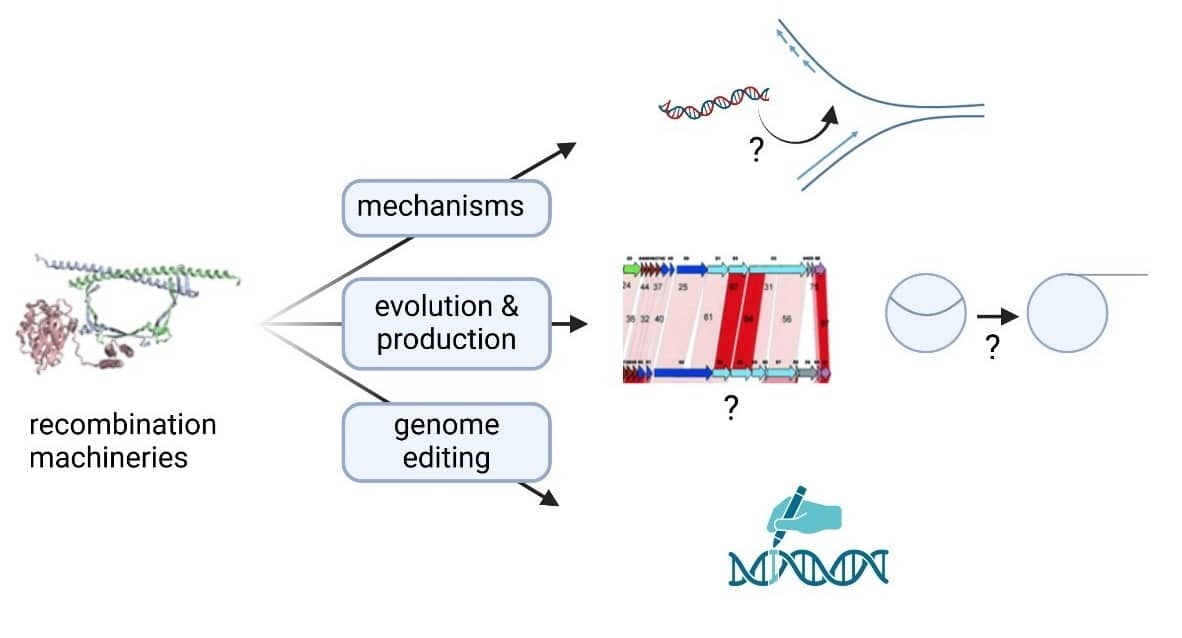
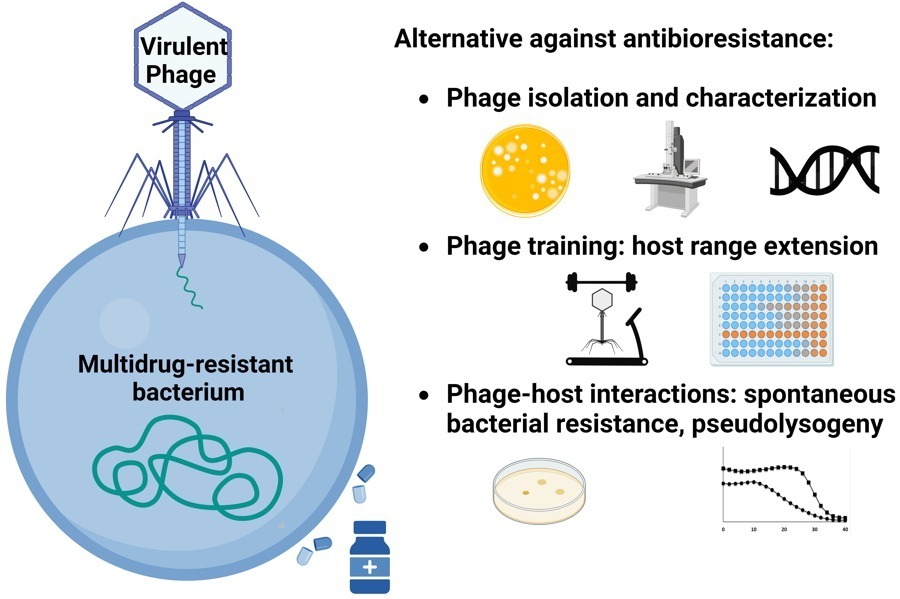
- Lossouarn, J.; Briet, A.; Moncaut, E.; Furlan, S.; Bouteau, A.; Son, O.; Leroy, M.; DuBow, M.S.; Lecointe, F.; Serror, P.; Petit, M.-A. Enterococcus faecalis Countermeasures Defeat a Virulent Picovirinae Bacteriophage. Viruses 2019, 11, 48. doi:10.3390/v11010048
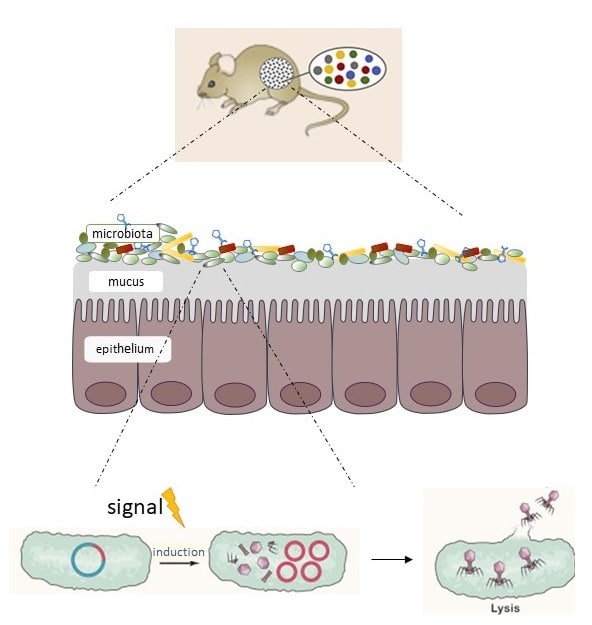
- Mathieu A, Dion M, Deng L, Tremblay D, Moncaut E, Shah SA, Stokholm J, Krogfelt KA, Schjørring S, Bisgaard H, Nielsen DS, Moineau S, Petit MA. Virulent coliphages in 1-year-old children fecal samples are fewer, but more infectious than temperate coliphages. Nat Commun. 2020 Jan 17;11(1):378. doi:10.1038/s41467-019-14042-z.
- Billaud, M., Lamy-Besnier, Q., Lossouarn, J., Moncaut, E., Dion, M.B., Moineau, S., Traoré, F., Le Chatelier, E., Denis, C., Estelle, J., Achard, C., Zemb, O., Petit, M.-A. Analysis of viromes and microbiomes from pig fecal samples reveals that phages and prophages rarely carry antibiotic resistance genes. ISME COMMUN. 1, 55 (2021). doi:10.1038/s43705-021-00054-8
- Misson P, Bruder E, Cornuault JK, De Paepe M, Nicolas P, Demarre G, Lakisic G, Petit MA, Espeli O, Lecointe F. Phage production is blocked in the adherent-invasive Escherichia coli LF82 upon macrophage infection. PLoS Pathog. 2023;19(2):e1011127. doi: 10.1371/journal.ppat.1011127.
- Terzian P, Olo Ndela E, Galiez C, Lossouarn J, Pérez Bucio RE, Mom R, Toussaint A, Petit MA, Enault F. PHROG: families of prokaryotic virus proteins clustered using remote homology. NAR Genom Bioinform. 2021 Aug 5;3(3):lqab067. doi: 10.1093/nargab/lqab067.
All the team publications are available in the DYNPHAGES-MICALIS HAL collection.